
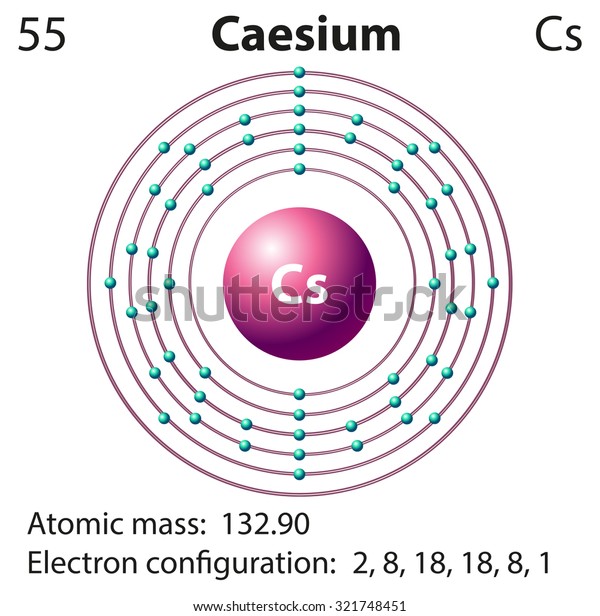
When their surfaces are cleaned, they are shiny. All the alkali metals show the typical properties of metals. This element is also radioactive, and not much is known about its chemistry because it is so scarce. Lithium, rubidium, and cesium are relatively rare, while francium is extremely rare, occurring only in trace amounts in the Earth’s crust. Sodium and potassium are relatively abundant in the Earth’s crust compared to the other alkali metals. Li is lithium, Na sodium, K potassium, Rb rubidium, Cs cesium, whose IUPAC spelling includes an A, and Fr francium. Let’s have a closer look at these elements. The alkali metals consist of Li, Na, K, Rb, Cs, and Fr. The alkali metals do not include hydrogen at the top of the group because hydrogen is not a metal but a nonmetal and a gas under standard conditions. They are called the alkali metals because all form alkaline solutions when they react with water. These metals are grouped together because they react in a similar manner.

The alkali metals is the name given to the group one elements on the periodic table. Let’s begin by having a look where the alkali metals are located on the periodic table. Lastly, we will investigate the reaction of the alkali metals with halogens, oxygen, and water and have a look at the equations for these reactions. We will have a look at the reactivity of the alkali metals and discuss why they react in the way that they do. Specifically, we will learn which elements are classified as alkali metals, where they are located on the periodic table, and the property trends in this group of elements. In this video, we will learn about the alkali metals.


 0 kommentar(er)
0 kommentar(er)
